

KEYTRUDA® (pembrolizumab), as a single agent, is indicated for adjuvant treatment following resection and platinum-based chemotherapy for adult patients with stage IB (T2a ≥4 cm), II, or IIIA non–small cell lung cancer (NSCLC).

IO = immunotherapy; PD-L1 = programmed death ligand 1.
DISEASE RECURRENCE CAN OCCUR, EVEN AFTER RESECTION, WITH OR WITHOUT CHEMOTHERAPY
Rate of Recurrence or Death Within 5 Years by Stage1
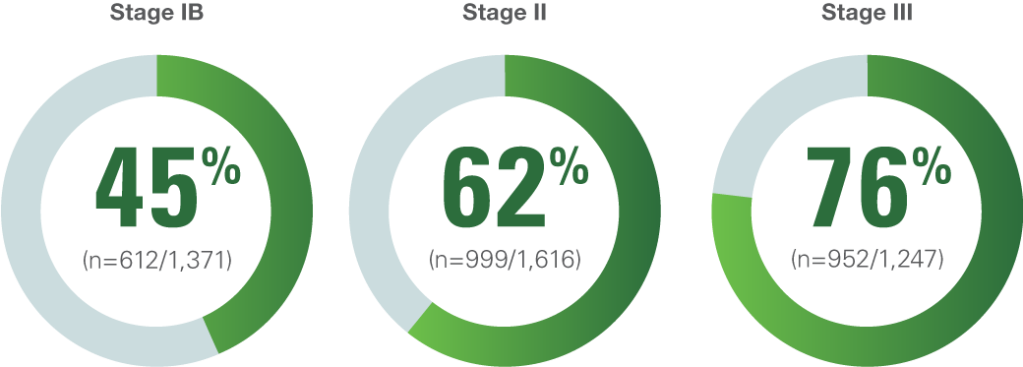
The rate of recurrence or death is based on a 2008 pooled analysis of 4,584 patients across 5 randomized trials, after complete resection, with or without adjuvant chemotherapy. The trials included in this pooled analysis occurred from 1994–2001.
Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group1
The Lung Adjuvant Cisplatin Evaluation study was a pooled analysis of 5 randomized trials conducted by the LACE Collaborative Group. The study evaluated the use of cisplatin-based chemotherapy as an adjuvant treatment for patients with NSCLC. The primary end point was overall survival (OS) and a secondary end point was disease-free survival (DFS).
Study population1
Individual patient data were collected and pooled from 5 trials including 4,584 patients who underwent complete resection. Of these patients, 2,281 received adjuvant chemotherapy. The interactions between patient subgroups or treatment types and chemotherapy effect on OS were analyzed using hazard ratios and log-rank tests stratified by trial.
Inclusion and exclusion criteria1
Trials eligible for inclusion were those that randomly assigned more than 300 patients with completely resected NSCLC to receive postoperative cisplatin-based chemotherapy versus no chemotherapy or cisplatin-based chemotherapy plus postoperative radiotherapy (administered sequentially) versus postoperative radiotherapy alone.
KEYNOTE-091: A MULTICENTER, RANDOMIZED, TRIPLE-BLIND, PLACEBO-CONTROLLED TRIAL IN PATIENTS WITH RESECTED STAGE IB (T2a ≥4 cm), II, OR IIIA NSCLCa

- Primary efficacy outcome measure: DFSd in the overall population.
- Additional efficacy outcome measure was OS in the overall population.
aAs defined per AJCC 7th ed. b Randomization was stratified by stage (IB vs II vs IIIA), adjuvant chemotherapy (no adjuvant chemotherapy vs adjuvant chemotherapy), PD-L1 status (TPS < 1% [negative] vs TPS 1%–49% vs TPS ≥50%), and geographic region (Western Europe vs Eastern Europe vs Asia vs rest of world).
cRECIST v1.1–defined disease recurrence as determined by the investigator.
dInvestigator-assessed DFS was defined as the time between the date of randomization and the date of first recurrence (local/regional recurrence, distant metastasis), appearance of a second NSCLC primary or other malignancy, or death from any cause, whichever occurred first.2
AJCC = American Joint Committee on Cancer; Q3W = every 3 weeks; TPS = tumor proportion score; RECIST v1.1 = Response Evaluation Criteria In Solid Tumors v1.1.
KEYNOTE-091: A MULTICENTER, RANDOMIZED, TRIPLE-BLIND, PLACEBO-CONTROLLED TRIAL IN PATIENTS WITH RESECTED STAGE IB (T2a ≥4 cm), II, OR IIIA NSCLCa
Of 1,177 patients randomized, 1,010 (86%) received adjuvant platinum-based chemotherapy following resection.

a As defined per AJCC 7th ed.
ECOG PS = Eastern Cooperative Oncology Group performance status.
In patients with stage IB (T2a ≥4 cm), II, or IIIA NSCLC, after resection and adjuvant platinum-based chemotherapy, regardless of PD-L1 expression,
KEYTRUDA DEMONSTRATED A CLINICALLY MEANINGFUL IMPROVEMENT IN DFS COMPARED TO PLACEBO

- In an exploratory subgroup analysis of the 167 patients (14%) who did not receive adjuvant chemotherapy, the DFS HR was 1.25 (95% CI, 0.76–2.05).
- At the time of the interim analysis, OS results were not mature (42% with events in the overall population).
Median DFS for Patients Who Received Adjuvant Chemotherapy

aHR based on the unstratified univariate Cox regression model.
b4.891 years (58.7 months).
c2.908 years (34.9 months).
HR = hazard ratio; CI = confidence interval; NR = not reached.
Before prescribing KEYTRUDA*, please refer to the full prescribing information by
Before prescribing KEYTRUDA*, please refer to the full selected safety information by